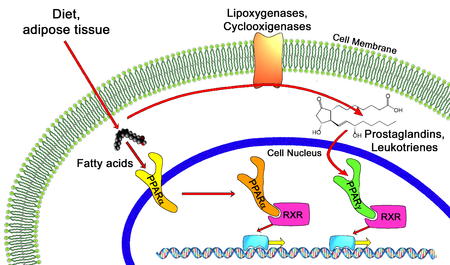
PPAR -alpha and -gamma pathways.
Contents |
Nomenclature and tissue distribution
| Peroxisome proliferator-activated receptor alpha | |
|---|---|
| Identifiers | |
| Symbol | PPARA |
| Alt. symbols | PPAR |
| Entrez | 5465 |
| HUGO | 9232 |
| OMIM | 170998 |
| RefSeq | NM_001001928 |
| UniProt | Q07869 |
| Other data | |
| Locus | Chr. 22 q12-q13.1 |
| Peroxisome proliferator-activated receptor gamma | |
|---|---|
| Identifiers | |
| Symbol | PPARG |
| Entrez | 5468 |
| HUGO | 9236 |
| OMIM | 601487 |
| RefSeq | NM_005037 |
| UniProt | P37231 |
| Other data | |
| Locus | Chr. 3 p25 |
| Peroxisome proliferator-activated receptor delta | |
|---|---|
| Identifiers | |
| Symbol | PPARD |
| Entrez | 5467 |
| HUGO | 9235 |
| OMIM | 600409 |
| RefSeq | NM_006238 |
| UniProt | Q03181 |
| Other data | |
| Locus | Chr. 6 p21.2 |
Three types of PPARs have been identified: alpha, gamma, and delta (beta):[3]
- α (alpha) - expressed in liver, kidney, heart, muscle, adipose tissue, and others
- β/δ (beta/delta) - expressed in many tissues but markedly in brain, adipose tissue, and skin
- γ (gamma) - although transcribed by the same gene, this PPAR through alternative splicing is expressed in three forms:
- γ1 - expressed in virtually all tissues, including heart, muscle, colon, kidney, pancreas, and spleen
- γ2 - expressed mainly in adipose tissue (30 amino acids longer)
- γ3 - expressed in macrophages, large intestine, white adipose tissue.
History
PPARs were originally identified in Xenopus frogs as receptors that induce the proliferation of peroxisomes in cells.[5] The first PPAR (PPARα) was discovered during the search of a molecular target for a group of agents then referred to as peroxisome proliferators, as they increased peroxisomal numbers in rodent liver tissue, apart from improving insulin sensitivity.[6] These agents, pharmacologically related to the fibrates were discovered in the early 1980s. When it turned out that PPARs played a much more versatile role in biology, the agents were in turn termed PPAR ligands. The best-known PPAR ligands are the thiazolidinediones; see below for more details.After PPARδ (delta) was identified in humans in 1992,[7] it turned out to be closely-related to the PPARβ (beta) previously described during the same year in other animals (Xenopus). The name PPARδ is generally used in the US, whereas the use of the PPARβ denomination has remained in Europe where this receptor was initially discovered in Xenopus.
Physiological function
All PPARs heterodimerize with the retinoid X receptor (RXR) and bind to specific regions on the DNA of target genes. These DNA sequences are termed PPREs (peroxisome proliferator hormone response elements). The DNA consensus sequence is AGGTCANAGGTCA, with N being a random nucleotide. In general, this sequence occurs in the promotor region of a gene, and, when the PPAR binds its ligand, transcription of target genes is increased or decreased, depending on the gene. The RXR also forms a heterodimer with a number of other receptors (e.g., vitamin D and thyroid hormone).The function of PPARs is modified by the precise shape of their ligand-binding domain (see below) induced by ligand binding and by a number of coactivator and corepressor proteins, the presence of which can stimulate or inhibit receptor function, respectively.[8]
Endogenous ligands for the PPARs include free fatty acids and eicosanoids. PPARγ is activated by PGJ2 (a prostaglandin). In contrast, PPARα is activated by leukotriene B4. PPARγ activation by agonist RS5444 may inhibit anaplastic thyroid cancer growth.[9]
Genetics
The three main forms are transcribed from different genes:- PPARα - chromosome 22q12-13.1 (OMIM 170998)
- PPARβ/δ - chromosome 6p21.2-21.1 (OMIM 600409)
- PPARγ - chromosome 3p25 (OMIM 601487).
Structure
Like other nuclear receptors, PPARs are modular in structure and contain the following functional domains:- (A/B) N-terminal region
- (C) DBD (DNA-binding domain)
- (D) flexible hinge region
- (E) LBD (ligand binding domain)
- (F) C-terminal region
Pharmacology and PPAR modulators
Main article: PPAR modulator
PPARα and PPARγ are the molecular targets of a number of marketed drugs. For instance the hypolipidemic fibrates activate PPARα, and the anti diabetic thiazolidinediones activate PPARγ. The synthetic chemical perfluorooctanoic acid activates PPARα while the synthetic perfluorononanoic acid activates both PPARα and PPARγ. Berberine activates PPARγ.